Cluster 1:
Signals derived from dying cells
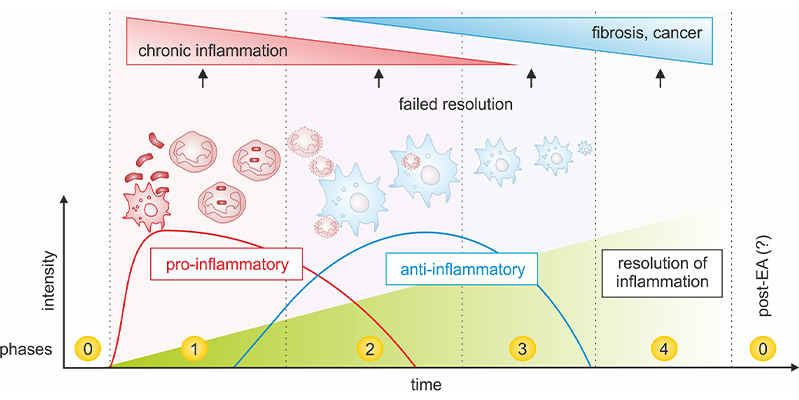
Cell Death is central during inflammation. Immune cells die after activation by pathogen-derived mediators, while other cells loose viability in response to exogenous (paracetamol and other drugs) or endogenous noxes (Fas/FasL, hypoxia). Different modes of cell death are known, including apoptosis, necrosis, or necroptosis. It is not understood how the resolution of inflammation is differentially influenced by these types of cell death. Cluster 1aims to answer this question and comprises three projects:
Project 1:Mechanisms of IL-38 signaling that promote the resolution of inflammation
IL-1 family proteins are critical regulators of inflammation. The new IL-1 family receptor antagonist IL-38, which is released from apoptotic cells, promotes the resolution of inflammation partly due to its impact on pathogenic T cells such as γδ T cells. The project explores other mechanisms of the pro-resolving nature of IL-38, including its impact on the generation of auto-antibodies, and investigates the so-far elusive molecular mechanisms of IL-38 signaling. To achieve these goals in vitro models of immune cell activation, in vivo inflammation models, and patient material will be employed.
Principle Investigators:
Prof. Dr. Andreas Weigert
Institute of Biochemistry 1
Goethe-University Frankfurt
Website
Project 3:The interplay between inflammation resolution and regeneration in acetaminophen-induced liver injury
Acetaminophen (APAP)-induced acute liver injury (ALI) not only is a continual clinical problem but as experimental protocol a well-established paradigmatic model of drug-induced necroinflammation. Recent studies imply that inflammatory signals not only mediate tissue damage but may also set the stage for hepatic regeneration e.g. by inducing pro-proliferative IL-6. In this PhD project we aim to further characterize parameters that drive hepatic regeneration during APAP intoxication by investigating their interplay with moderate and severe forms of the associated necroinflammatory response.
Principle Investigator:
Prof. Dr. Heiko Mühl
Institute of General Pharmacology and Toxicology
Goethe-University Frankfurt
Website
Project 13:Role of TAM receptors in responding to proinflammatory forms of cell death
Recognition and clearance of phosphatidylserine (PS)-expressing apoptotic cells by efferocytosis alters phagocytes activation and promotes the resolution of inflammation (ROI) thereby leading to the establishment of an immune suppressive environment. In acute leukemia, we found that targeting PS-recognition receptors on phagocytes, promotes protective inflammation and elicits anti-leukemic immunity. Because impairment of apoptosis frequently occurs in therapy resistant cancers, in this project we will (1) evaluate the immunological consequences of inducing alternative pro-inflammatory forms of cell death and (2) explore the in vivo therapeutic potential of combined blockade of TAM receptors on phagocytes and cell-death induction using pre-clinical models of highly aggressive cancers.
Principle Investigator:
Dr. Hind Medyouf
Georg- Speyer-Haus
Website
Cluster 2:
Polarization of immune cells
Phagocytosis of dying cells, cell debris or pathogens by macrophages and mast cells, is an important process during resolution of inflammation. The projects within cluster 2 deal with polarization of phagocytes induced by endogenous and exogenous signals and explore signal transduction pathways activated or inhibited during phagocyte-induced resolution of inflammation. Cluster 2 comprises five projects:
Project 4:Fatty acid provision upstream of cPLA2α during the resolution of inflammation in leukocytes
The central enzymes involved in lipid mediator formation in human leukocytes during the time course of inflammation such as leukotrienes, prostaglandins and specialized pro-resolving mediators (SPM) are well characterized. In contrast, little is known about the cellular supply (uptake, transport, storage and intracellular release) of the polyunsaturated fatty acids that serve as precursors to these bioactive lipids. During the transition from inflammation to its resolution, a change in the macrophage phenotype and thus a change in the lipid mediators released in the inflamed tissue takes place: The formation of pro-inflammatory, arachidonic acid-based oxylipins is attenuated in favor of omega-3-based ones. It is so far not known which factors control this switch in precursor fatty acids. In this project, we are therefore interested in the identification and characterization of new players upstream of the known biosynthetic pathways that control this precursor switch during inflammation resolution in macrophages.
Project 5:Role of soluble epoxide hydroxylase (sEH) in macrophage polarization and resolution of Inflammation
The expression/activity of the soluble epoxide hydrolase (sEH) is important for optimal progenitor cell proliferation and long-term sEH inhibition is detrimental to progenitor cell proliferation, mobilization and vascular repair. We hypothesize that the sEH plays an important role in the regulation of myeloid cell function and aim to determine the role of the sEH in regulating macrophage polarization, lipid storage and foam cell development in the frame of atherosclerosis. Use will be made of available proteomic and metabolomic approaches, pharmacological tools as well as siRNA/CRISPR approaches and genetic mouse models e.g. the myeloid-specific deletion of the sEH.
Principle Investigator:
Prof. Dr. Ingrid Fleming
Institute for Vascular Signalling
Goethe University Frankfurt
Website
Project 6:Translational regulation of the resolution of inflammation in macrophages
Gene expression changes during the course of inflammation and its resolution are rather well established. There is ample evidence that especially during the resolution phase RNA expression changes are strongly controlled on a post-trancriptional level. In the present project, we aim to elucidate the translational changes in macrophages during the resolution of inflammation and characterize the functional impact of translational changes on the course of the resolution phase.
Project 7:Pro-resolving mediators in mast cells
Mast cells are known for their proinflammatory properties i.e. in promoting allergies. However, they have also the power to suppress immune answers and to promote resolution of inflammation. Here, we aim to identify signals that induce the selective release of antiinflammatory and pro-resolving mediators from mast cells as well as the downstream effector cells targeted by these mediators.
Principle Investigator:
Prof. Dr. Klaus Scholich
Institute of Clinical Pharmacology
Goethe-University Frankfurt
Website
Project 8:The impact of IKKe inhibition on the velocity of atherosclerosis progression: Do T-cells play a role?
In a running PhD project of the graduate school, we investigate the role of IKKe in inflammatory processes of peritonitis and atherosclerosis in mice. Our current results indicate that a deletion of IKKe ameliorates the inflammatory symptoms and leads to an improved health status. Interestingly, by examining immune cells in IKKe knock-out and wild type mice we detected a significantly increased level of specialized T-cells in several tissues of IKKe-depleted mice. In a follow-up project, we now intend to investigate 1. if the effects of IKKε deletion can be mimicked by the specific IKKe inhibitor drug amlexanox and if this drug can enhance the resolution of inflammation, 2. if IKKe deletion in mice slows down the progression of atherosclerosis and 3. if T-cell subsets play a role in IKKe-mediated effects on inflammatory processes.
Principle Investigator:
Prof. Dr. Ellen Niedernberger
Institute of Clinical Pharmacology
Goethe-University Frankfurt
Website
Project 12:SARS-CoV-2 infections in monocytes/macrophages: inflammatory patterns and resolution of inflammation
Systemic and local inflammation associated with organ damage are critical determinants of morbidity and mortality in COVID-19. Several types of immune cells have been proposed as drivers of site-specific inflammation, however, the SARS-CoV-2-induced inflammation patterns in these cells are yet to be determined. This project aims to investigate mechanism of inflammation and its resolution in SARS-CoV-2 infected monocytes/ macrophages and determine if these pathways may prove as a therapeutic target to treat COVID-19 by resolving locally induced inflammation.
Principle Investigator:
Prof. Dr. Denisa Bojkova
Institute of Virology
University Hospital Frankfurt
Website
Cluster 3:
Restoration of barrier function
A key process during inflammation is the loss of epithelial and endothelial barrier function. This enables immune cells to invade the subendothelial space or allows noxious substances to enter the body and trigger immune reactions. Restoration of barrier function is a fundamental principle during the resolution of inflammation. Regeneration of biological barriers requires a crosstalk between cells of the barrier and (anti-inflammatory) immune cells. Underlying signalling pathways are analysed within the three projects of cluster 3:
Project 9:Characterization of the kinase inhibitor C81 and of its targets CLKs/DYRKs for the protection of barriers as strategy to enhance the resolution of inflammation
Chronic inflammation is characterized by a disturbed resolution of inflammation, in which the barrier function of the vascular endothelium for leukocytes is compromised and a dysregulation of angiogenic processes is observed. In the first application period, we were able to show that the substance C81, which is derived from a natural product, reduces the leukocyte-endothelial interaction and strengthens the endothelial barrier by influencing endothelial activation processes. Further work has shown that C81 also influences leukocytes and inhibits angiogenesis-associated processes in endothelial cells. The target structures of C81 are kinases, in particular CLKs and DYRKs. The role of these kinases in leukocytes and endothelial cells with respect to processes triggering the resolution of inflammation is unknown. The aims of the follow-up application are therefore 1) to investigate whether and how C81 and the inhibition of CLKs/DYRKs contribute to the resolution of inflammation by restoring a quiescent, angiogenetically inactive endothelium and 2) to analyze the mechanisms of action of C81 and of the inhibition of CLKs/DYRKs in leukocytes with regard to both leukocyte-endothelial interaction and processes associated with the resolution of inflammation (especially in macrophages).
Principle Investigator:
Prof. Dr. Robert Fürst
Institute of Pharmaceutical Biology
Goethe-University Frankfurt
Website
Project 10:Role of resolution of inflammation during cardiovascular organ remodeling
Phenotypic changes in organs in response to stress or adaptation are accompanied by inflammation, which facilitates epigenetic plasticity. Resolution of inflammation is therefore necessary to stabilize the cellular phenotype and to reinstate stable gene expression programs. It is not known whether the latter effects are dependent on active inflammation resolving mechanism. This aspect will be studied in models for cardio-vascular disease pathologies.
Principle Investigator:
Prof. Dr. Ralf P. Brandes
Institute of Cardiovascular Physiology
Goethe University Frankfurt
Website
Project 11:Reg3b in regeneration and cellular transformation
Using a new transgenic model of Reg3b overexpression in the intestine, we could observe a profound effect of Reg3b in wound healing in an acute model of colitis. Current efforts aim to characterize the exact molecular mechanisms that are controlled by Reg3b with a particular focus on changes in the tumor microenvironment. Moreover, we will elucidate the impact of Reg3b on sporadic intestinal tumorigenesis.
Principle Investigator:
Prof. Dr. Florian Greten
Georg-Speyer-Haus
Website